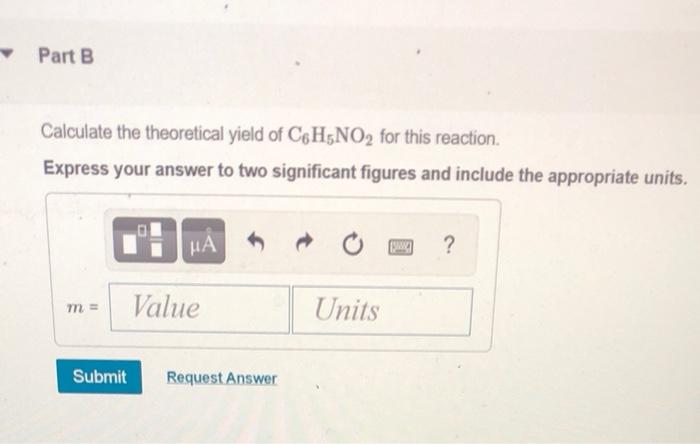
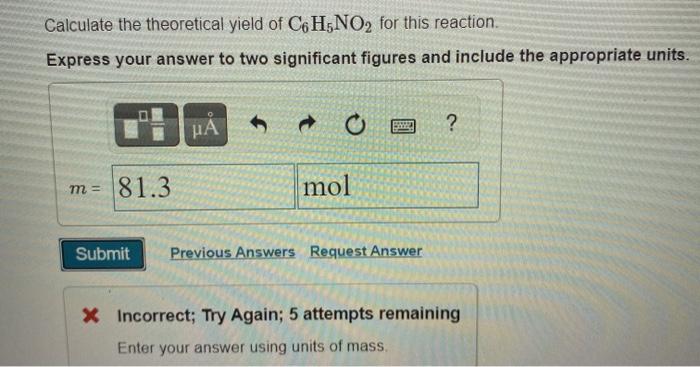
Calculate the Theoretical Yield of C6h5no2 for This Reaction
Mass of product molecular weight of product moles of limiting reagent in reaction stoichiometry of product where. So to stop you from wondering how to find theoretical yield here is the theoretical yield formula.
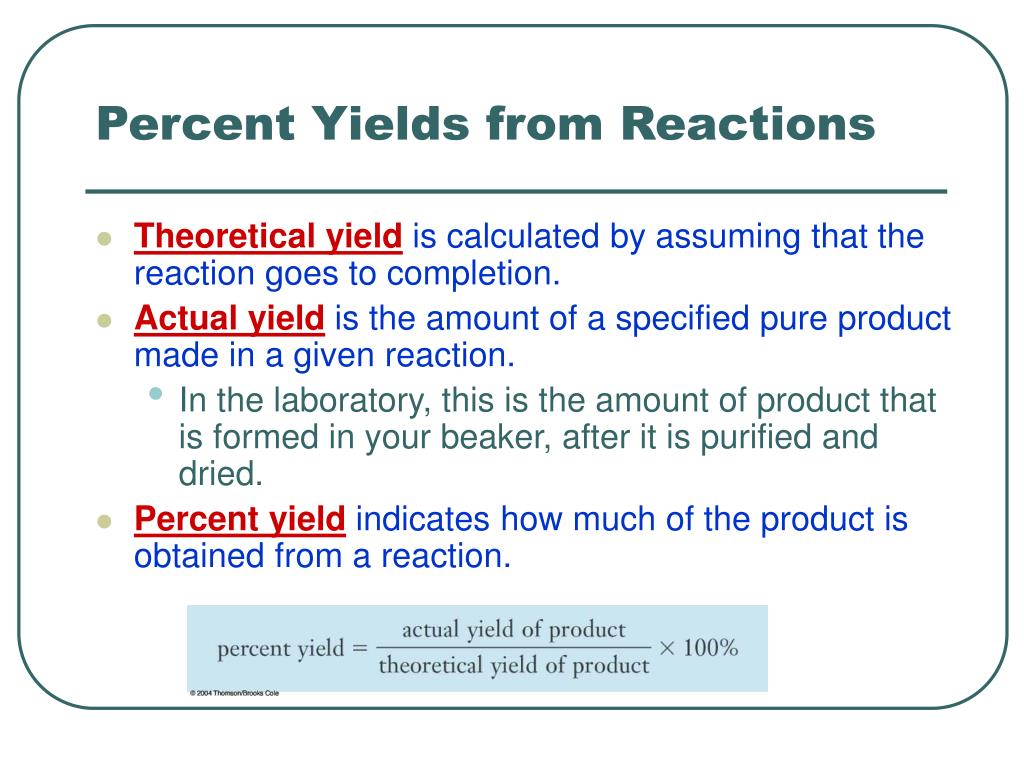
Ppt Percent Yields From Reactions Powerpoint Presentation Free Download Id 4980141
The formula for calculating the percent yield is.
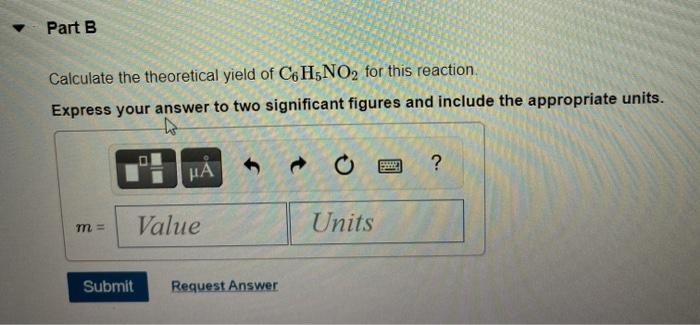
. TextPercent Yield fractextActual YieldtextTheoretical Yield times 100. This chemistry video tutorial explains how to calculate the percent yield actual yield and theoretical yield of a product produced in a chemical reaction gi. You are given the masses of both the reactants 277 g HNO3 and 77 g C6H6.
Actual yield 149g. Molar mass of H 2 gas 2 grams. From the experiment we already know the ACTUAL YIELD 316 g.
Actual Yield Theoretical Yield Yield 200954 g 0735 148 g. See also our theoretical yield calculator for chemical reactions probably your next stop to finish the problem set. No other quantities needed.
Molar Mass of Nitrobenzene C6H5NO2. Lets assume that you obtained an actual yield of 850 grams. Grams product grams reactant x 1 mol reactantmolar mass of reactant x mole ratio productreactant x molar mass of product1 mol product The theoretical yield of our reaction is calculated using.
Rearrange the above formula to obtain theoretical yield formula. Percentage yield Actual yieldtheoretical yield x100. Percent yield Yield.
Theoretical yield 1567 g use the un-rounded number for the calculation. Use the molar mass of the product to convert moles product to grams of product. Percentage yield mass of actual yield mass of theoretical yield 100.
Up to 256 cash back calculate the theoretical yield of C6H5NO2 for this reaction. The formula for percentage yield is given by. Percentage yield of NaCl 8559.
To calculate percent yield you simply take actual yield 1099 grams of sodium bromide divided by the theoretical yield 1452 grams of sodium bromide. The molar mass of C6H5NO2 Nitrobenzene is. C6H5NO2 l H 2 Ol The limiting reagent is the reactant that limits the amount of product produced.
Divide number of AI moles by two since it takes four to make 2 AI2O3 Finally to get a theoretical yield youll need to multiply the number of moles of the product by the molecular weight of the product. Actual yield is said to be the mass of ammonia that is actually produce during the chemical reaction. What is the percent yield of C6H5NO2 in this reaction.
This is the reactant that produces fewer moles of the product when it completely reacts. Then the percent yield would be. Theoretical yield of ammonia NH3 is said to be the mass of product predicted by the balanced chemical equation for the reaction.
Use the percent yield equation below. Collected from the reaction what is the percent yield. 3999 g m o l 1452 g N a B r.
Determine the theoretical yield of the formation of geranyl formate from 375 g of geraniol. List other known quantities and plan the problem. 1275 g C6H6 1 mol C6H6 7811 g C6H6 1 mol C6H5NO2 1 mol C6H6 12311 g C6H5NO2 1 mol C6H5NO2 200954 g C6H5NO2 Apply percent yield.
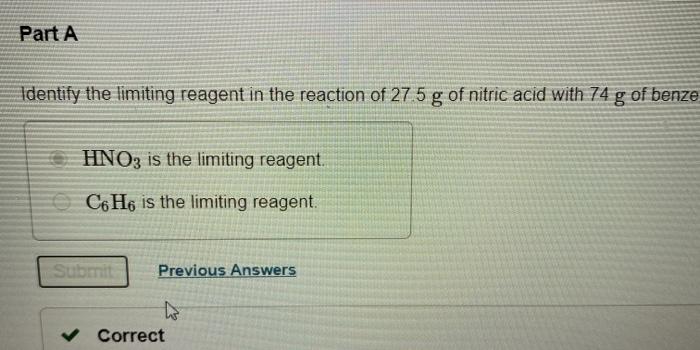
A reaction yield is reported as the percentage of the theoretical amount. C6H6 HNO3 C6H5NO2 H2O. The reaction involves two reactants C6H6 and HNO3 that react in a 11 ratio.
First use mass-mass stoichiometry conversion to determine theorectical yield of C6H5NO2. Moles of limiting reagent in reaction mass of limiting reagent molecular weight of limiting reagent stoichiometry of limiting reagent. 1099 g N a B r.
A 156g sample of C6H6 is mixed with excess HNO3. 05 moles of AI2O3 multiplied by the molecular weight of AI2O3 is approximately 10196 grams per mole. A chemist making geranyl formate.
Since the value of actual yield is usually less than the theoretical yield. Actual yield of ammonia NH3 408 g given in the above question 2. Once you convert 280 g HNOs and 74 g Cofle into the number of moles of each using their molar mass determine which produces less product Since the coeficientsn the balanced chemical equation are all 1 in this specific case you carn directly compare the number of.
Percentage yield of NaCl 850 grams 993 grams 100. We must CALCULATE the theoretical yield. We see there is expected to be 1452 grams of sodium bromide product.
224 g 316 g ACTUAL. We isolate 180g of C6H5NO2.

Oneclass Calculate The Theoretical Yield Of C6h5no2 For This Reaction Once You Convert 28 0 G Hnos A

How To Calculate Theoretical Yield 12 Steps With Pictures

How To Calculate Theoretical Yield 12 Steps With Pictures
5 3 Calculating Reaction Yields Problems Chemistry Libretexts
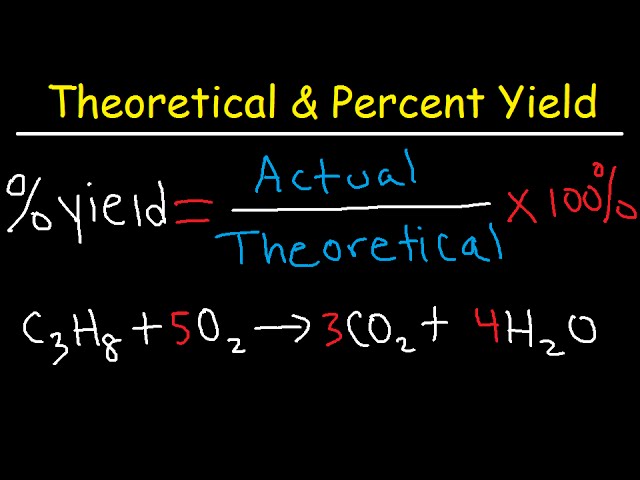
How To Calculate Theoretical Yield And Percent Yield Youtube

How To Calculate Theoretical Yields Youtube

How To Calculate Theoretical Yield 12 Steps With Pictures
Solved The Reaction It Is Asking About In The Picture Is Chegg Com

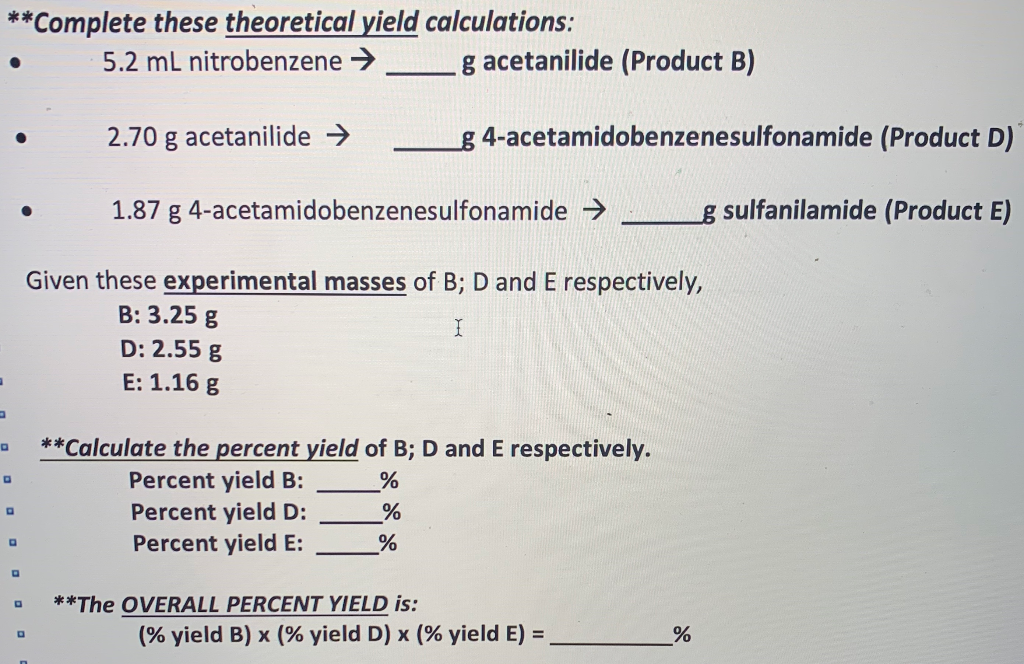
Solved Complete These Theoretical Yield Calculations 5 2 Chegg Com

How To Calculate Percent Yield In Chemistry Teaching Chemistry Physical Chemistry Chemistry

How To Calculate Theoretical Yield 12 Steps With Pictures

Solved Nitrobenzene Cshsno2 An Important Raw Material For Chegg Com
Solved Nitrobenzene C6h5no2 Is Used In Small Quantities As Chegg Com
Solved Complete These Theoretical Yield Calculations 5 2 Chegg Com
Calculate The Theoretical Yield Of C6h5no2 For This Chegg Com

Solved Nitrobenzene C6h5no2 Is Used In Small Quantities As Chegg Com
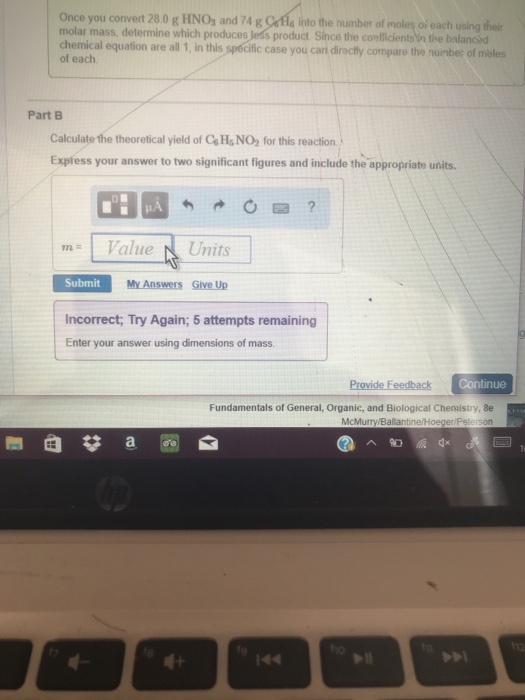
Solved Once You Convert 28 0 G Hnos And 74 G Cofle Into The Chegg Com
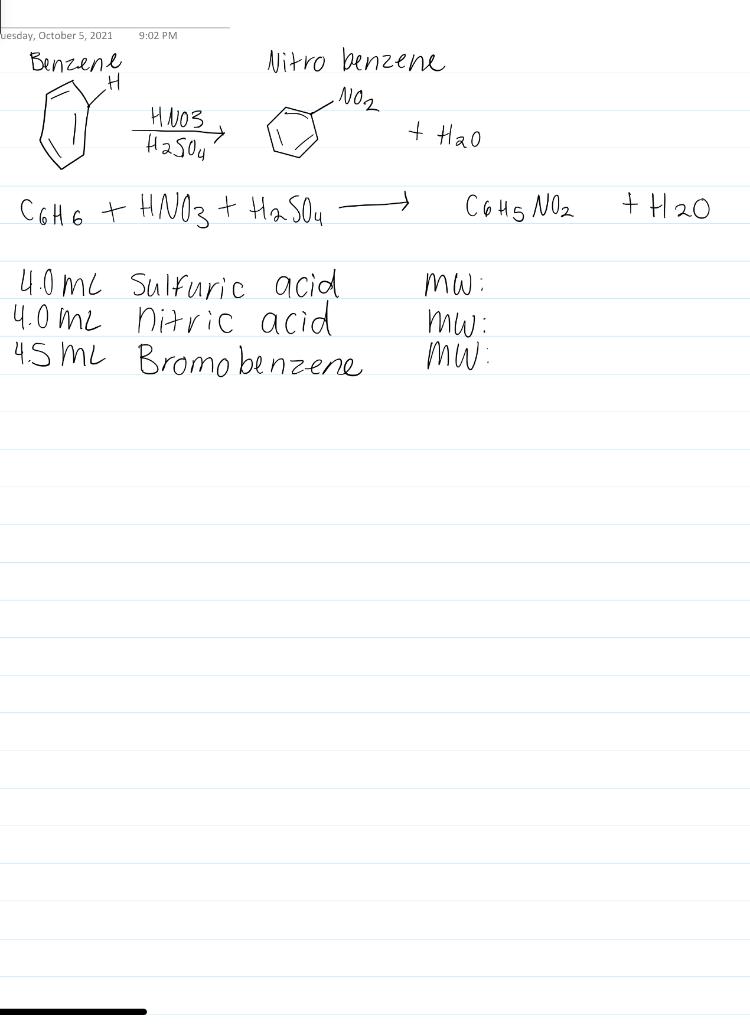
Calculate The Limiting Reactant And Theoretical Yield Chegg Com

Comments
Post a Comment